Dietary sugars, microbial acids and dental/gut disease – a deeper dive.

A look at how dietary sugars can negatively effect the dental and gut health of your pets.
Since as early as 1912, research has shown that in dogs, excessive quantities of glucose (such as rice, fruits, and honey) within their diet, together with meat, can lead to a variety of negative health consequences. For example, increased calcium oxalate crystal formation in the kidneys that are microscopically present in urine, which coincides with mucous gastritis – a condition that inflames the stomach lining.3 When excessive sugar is present in an animal’s diet, one of the waste products from microbial sugar fermentation is oxalic acid.5-6 Research from over the past 7 years shows that organic acids, like oxalic acid, can be produced by specific species of the dog’s oral and gut microbiomes that are linked to inflammation and chronic inflammatory diseases, such as gum disease. These organic acid fermentation products negatively impact your pet’s immune system. Dietary sugars can effect both cats and dogs, and result in changes within their overall dental and gut health.
From mouth to gut: the effect of dietary sugars on pets
When a cat or dog is over-exposed to dietary sugars (complex and simple sugars), such as rice, fruits, and honey, the structure and microbial composition of the dental plaque that exist on the tooth surface and under the gumline are significantly different.9,10 In a study on domestic and feral cats, domestic cats that ate commercial cat food had more calculus than feral cats who ate live prey.8 In our research, domestic cats on a commercial diet also have thicker plaque, (known to microbiologists as extracellular polymeric matrix, that contains extracellular polymeric substances, or EPS) with a course tartar and have more fungus and a higher burden of C. acnes and Peptostreptococcus and Streptococcus spp (destructive bacteria) than feral cats, which likely have a more diverse diet.
The EPS can be either protective or destructive in nature to the host, which is particularly influenced by diet. Diet influences the kinds of waste products produced by the oral microbiome and the identity of the microbes that inhabit the microbiome, and what kind of EPS they make. EPS examples made when sugar is available in the diet are oxalic acid, lactic acid, propionic acid, acetic acid and many others. The presence of these organic acids embedded in the plaque creates microbial communities that can reach a high acidity level, with a very low pH of 3.8-5.5. This induces inflammation in the gum tissue and areas of demineralization of tooth enamel, but also fosters the growth of acid-tolerant bacteria such as Enterococcus faecalis, Streptococcus spp and fungi.11 These microbes tend to be associated with inflammation and slowing the wound healing process, which is a significant problem in animals with gum disease and irritable bowel (IBD) – two common gut-related inflammatory processes driven by sugars in the diet.12
This transition from neutral or basic conditions in the mouth and lower gut to acidic conditions have a detrimental effect on the microbiome, also known as dysbiosis, which is an higher amount of dangerous bacteria over beneficial bacteria. A graphic description of the process is presented in Figure 1.
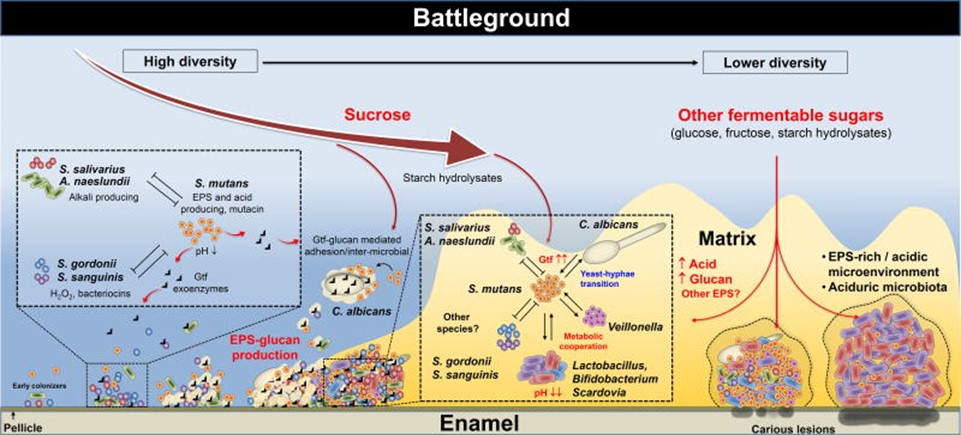
Preventive measures
Simple things that can be done to help dogs’ and cats’ upper and lower guts are:
- Restrict commercial diets with carbohydrates to only a day or two a week. Things like chickpeas, sweet potatoes, potatoes, rice, wheat, corn, honey, are quickly converted to simple sugars by oral and lower gut microbes, which can lead to inflammatory waste products.
- Limit treats and chews. Most commercial treats and chews have binding agents that are modified starches, which are very quickly converted to simple sugars.
- Opt for products whose major ingredients are protein – to steer the metabolic activity of the microbes towards a less-acidic profile. This profile will counterbalance the inflammatory acids produced by harmful dental bacteria.
References
- Mosenthal HO. 1911. Observations of the succus entericus. J. Exp. Med. 13(3):319-27.
- Grey EG. 1916. An experimental study of the effect of chole-cystgastrostomy on gastric acidity. J. Exp. Med. 23(1):15-24.
- King JH, Moyle RD, Haupt WC. 1912. Studies in glycosuria: second paper: glycosuria following anesthesia produced by the intravenous injection of ether. J. Exp. Med. 16(2):178-93.
- Marine D. 1914. Observations on tetany in dogs: relation of the parathyroids to the thyroid; relation of tetany to age, amount of parathyroid tissue removed, accessory parathyroids, pregnancy, lactation, rickets, sulphur, and diet; relation of parathyroids to sugar tolerance; effect of calcium salts. J. Exp. Med. 19(1):89-105.
- Hegedus DD, Rimmer SR. 2005. Sclerotinia sclerotiorum: When “to be or not to be” a pathogen? FEMS Microbiol Letters. 251(2):177-184.
- Li Z, Tongshuo B, Dai L, Wang F, Tao J, Meng S, Hu Y, Wang S, Hu S. 2016. A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Scientific Reports. (6):25313.
- Watson AD. 1994. Diet and periodontal disease in dogs and cats. Aust. Vet. J. 71(10):313-318.
- Clarke DE, Cameron A. 1998. Relationship between diet, dental calculus and periodontal disease in domestic and feral cats in Australia. Aust. Vet. J. 76(10):690-693.
- Takahashi N, Nyvad B. 2011. The role of bacteria in the caries process: ecological perspectives. J. Dent. Res. 90(3):294-303.
- Pitts NB, et al. 2017. Dental caries. Nat. Rev. Dis. Primers. 3:17030.
- Svensater G, Larsson UB, Greif EC, Cvitkovitch DG, Hamilton IR. 1997. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 12(5):266-73.
- Chong KK, Tay WH, Janela B, Yong AM, Liew TH, Madden L, Keogh D, Barkham TM, Ginhoux F, Becker DL, Kline KA. 2017. Enterococcus faecalis modulates immune activation and slows healing during wound infection. J. Infect. Dis. 216(12):1644-1654.
- Bowen WH, Burne RA, Wu H, Koo H. 2017. Oral biofilms: pathogens, matrix and polymicrobial interactions in microenvironments. Trends Microbiol. 26(3):229-242.





